Permanent gregarine specimen techniques
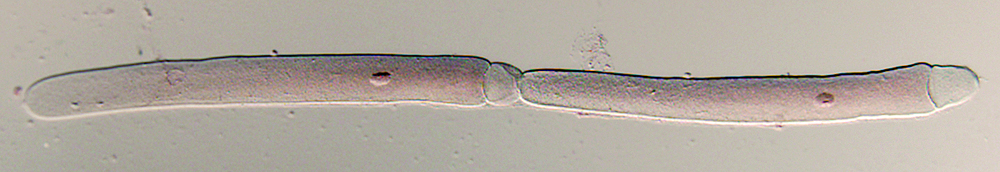
Working with parasitic protozoans requires a set of standardized techniques for making permanent specimens, especially for biodiversity discovery and taxonomic studies. The techniques presented here have been developed and refined over the last 20 years to enable us to make permanent museum specimens of gregarines. The techniques are adapted for use in the field and the laboratory, depending upon the source of insect hosts. Briefly, we make smears of host intestinal contents on clean 22mm coverslips, fix the specimens by flotation on hot AFA, and then store them in 80% ethanol in columbia jars. When we're in the field, we parafilm the columbia jars and do the final staining and mounting after returning to the lab. We use acetocarmine stain for routine work and depend upon DIC microscopy to elucidate details of epimerite morphology. It's a fast, easy, effective approach (see the banner image above). However, hematoxylin staining is particularly useful for elucidating details of very young trophozoites, epimerite morphology, and the structure of very small gregarine species. We use damar balsam as a mountant for all of our specimens. We find it preferable to canada balsam because damar balsam is more predictable by resin lot, less expensive, less acidic, and less prone to darkening over time than is canada balsam. Unlike permount and other synthetic resins, damar balsam is stable over time. We have a lot of specimens mounted in damar balsam over 20 years ago and there is no sign of deterioration or stain fade.
Use the links at right for details of our techniques or download the complete PDF.